Abstract
Introduction: Prognostic indexes have been widely used in oncology. International prognostic scoring system (IPSS) in myelodysplastic syndrome and Dynamic IPSS (DIPSS) in myelofibrosis play an important role in separating low- and high-risk patients (pts). In ALL, absence of prognostic index limits clinicians ability to risk stratify patients. Our goal is to create a prognostic index for survival in pts with Philadelphia-negative B-cell ALL incorporating minimal residual disease status (MRD) in addition to baseline clinical features.
Methods: We reviewed 419 untreated patients with Philadelphia-negative B-cell ALL who received hyper-CVAD-based regimens (n=327) or augmented BFM (n=92) between April 2000 and April 2015. MRD assessment at the time of complete remission (CR) was included in the analysis. MRD testing was performed by multi-colored flow cytometry with a lowest detection level of 10-4.
Cox proportional hazards regression models were fit to assess the association between survival and clinical characteristics of pts with available MRD data at the time of CR (n=276). We constructed the prognostic index on the basis of the independent prognostic factors included in the final multivariable Cox model. We assigned a weighted risk score to each factor based on the regression parameters from the Cox regression analysis.
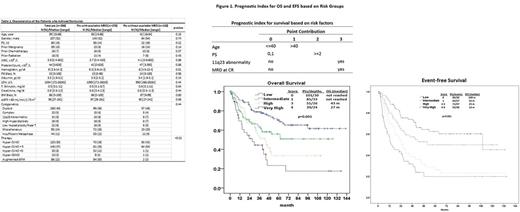
Results: Overall, 394 pts (94%) achived complete response (CR), 13 (3%) were refractory, and 12 (3%) died before response assessment. MRD data was available in 276 (70%) of the responding pts and it was missing for 118 (30%) pts at the time of CR. MRD assessments were missing mostly for pts who were treated in 2004 or earlier; 93 of 118 pts (79%). Median time to MRD evaluation was 24 days [range, 13-145]. Clinical characteristics of pts with or without available MRD data were similar except treatment regimens (Table 1). Overall, 186 pts (67%) achieved MRD negativity and 90 (33%) were MRD positive at CR. With a median follow-up of 75 months (range, 65 to 87), 142 (51.4%) patients had events (relapse, death, secondary leukemia). The median event-free survival (EFS) was 38.9 months (95% CI: 28.7 - 53.7) and following factors were associated with inferior EFS; performance status (PS) ≥2, positive MRD at CR, age >40 and presence of 11q23 chromosomal abnormality. Among the total 276 patients, 113 patients died (41%). The median OS was 67 months (95% CI: 49 - not estimable); positive MRD at CR and age > 40 were the only two factors that were significantly associated with death.
We developed a simplified prognostic index for survival using statistically significant clinical risk factors identified in the multivariate analysis (Figure 1). The prognostic index score is calculated by summing the total of risk factors with points given for age, 11q23 chromosomal abnormality, PS≥2, and MRD status at CR. The model was able to separate four distinct groups; low, intermediate, high and very-high risk.
In total, 35 patients (13%) underwent allogeneic stem cell transplant (ASCT) in CR1 (median time to ASCT - 135 days). A landmark analysis at 4.5 months showed that ASCT in CR1 was associated with inferior survival in low- and intermediate-risk patients; median OS was not reached in patients who did not receive ASCT and it was 95 months among ASCT recipients (p=0.034). High-and very high-risk patients had similar survival regardless of receiving ASCT in first CR or not; 43 months and 45 months, respectively (p=0.439).
CONCLUSIONS: We defined four distinct risk groups for survival and demonstrated that MRD status at CR is the strongest factor predicting OS. ASCT in first CR may not improve survial in low- and intermediate-risk patients. Our findings require validation on a separate cohort of patients.
Kantarjian: Pfizer: Research Funding; Bristol-Meyers Squibb: Research Funding; ARIAD: Research Funding; Amgen: Research Funding; Novartis: Research Funding; Delta-Fly Pharma: Research Funding. Khoury: Stemline Therapeutics: Research Funding; Pfizer: Research Funding; Angle: Research Funding; Kiromics: Research Funding. Cortes: Sun Pharma: Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; Teva: Research Funding; Pfizer: Consultancy, Research Funding; ARIAD: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; BMS: Consultancy, Research Funding. Wierda: The University of Texas MD Anderson Cancer Center: Employment; Emergent: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Juno: Research Funding; Sanofi: Consultancy, Honoraria; Genzyme: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Acerta: Research Funding; Janssen: Research Funding; Genentech/Roche: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria; GSK/Novartis: Consultancy, Honoraria, Research Funding; Karyopharm: Research Funding; Kite: Research Funding. Jain: Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Verastem: Research Funding; Celgene: Research Funding; Genentech: Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Verstovsek: CTI BioPharma Corp: Research Funding; Seattle Genetics: Research Funding; Lilly Oncology: Research Funding; Pfizer: Research Funding; Galena BioPharma: Research Funding; Genentech: Research Funding; Blueprint Medicines Corp: Research Funding; Bristol Myers Squibb: Research Funding; Roche: Research Funding; Roche: Research Funding; Pfizer: Research Funding; Incyte: Research Funding; Lilly Oncology: Research Funding; Blueprint Medicines Corp: Research Funding; Celgene: Research Funding; NS Pharma: Research Funding; Genentech: Research Funding; NS Pharma: Research Funding; Gilead: Research Funding; Astrazeneca: Research Funding; Incyte: Research Funding; Astrazeneca: Research Funding; Bristol Myers Squibb: Research Funding; Promedior: Research Funding; CTI BioPharma Corp: Research Funding; Galena BioPharma: Research Funding; Promedior: Research Funding; Gilead: Research Funding; Celgene: Research Funding; Seattle Genetics: Research Funding. O'Brien: Gilead Sciences, Inc.: Consultancy, Other: Research Support: Honorarium, Research Funding; Acerta: Other: Research Support: Honorarium, Research Funding; Vaniam Group LLC: Consultancy; Pfizer: Consultancy, Research Funding; Sunesis: Consultancy; Aptose Biosciences, Inc.: Consultancy; Janssen: Consultancy; TG Therapeutics: Consultancy, Other: Research Support: Honorarium, Research Funding; Celgene: Consultancy; Pharmacyclics: Consultancy, Other: Research Support: Honorarium, Research Funding; ProNAI: Other: Research Support: Honorarium, Research Funding; Astellas: Consultancy; AbbVie: Consultancy; Amgen: Consultancy; CLL Global Research Foundation: Membership on an entity's Board of Directors or advisory committees; Regeneron: Other: Research Support: Honorarium, Research Funding; GSK: Consultancy; Alexion: Consultancy. Jabbour: Bristol-Myers Squibb: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal